Understanding the function of enzymes, which are the catalysts nature developed over long time during evolution, is a key to the design of new, very specific, and active catalysts – one of UniSysCat‘s research aims. In their recent study published in Science, a team from the UniSysCat groups of Holger Dobbek (HU Berlin), Petra Wendler (Uni Potsdam) and Athina Zouni (HU Berlin) shed light on the structure and function of photosystem II (PSII). This enzyme complex plays a crucial role in photosynthesis. By using cryo-electron microscopy as a visualization technique, they gain structural insights with the highest resolution ever achieved and thus clarify important aspects of the function of PSII.
PSII is part of the photosynthetic apparatus of plants, algae and cyanobacteria. It starts the photosynthetic reaction chain that converts solar energy into chemical energy and thus sustains life on Earth. Using solar light as an energy source and catalysts based on earth-abundant elements, it can split water and store energy. PSII thus serves as a blueprint for emerging technologies for the sustainable production of fuels and chemicals. Researchers around the world are, therefore, trying to precisely retrace the reactions within the large molecule and learn from them.
The chemical reactions that take place within PSII are very precisely tuned to its structure, the charge state, and the interactions between subunits of the large molecule. The smallest changes, e.g. through dehydration or mutation of critical amino acids, can reduce the efficiency of PSII and can even impair its function.
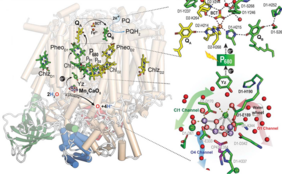
Up until now, studies of the structure of PSII and its function have been primarily based on X-ray diffraction. The structure could thus be determined at resolutions of 1.85 Å (synchrotron) and 1.89 Å (XFEL). However, certain functional features of PSII remained speculative since X-ray techniques cannot well resolve the positions of hydrogen atoms and protons within PSII. However, cryo-electron microscopy can provide information about these light elements like protons and hydrogens, as the UniSysCat team demonstrated in their recent study.
The team could visualize the structure of PSII of cyanobacterium Thermosynechococcus vestitus at the unprecedented resolution of 1.71 Å. Their analysis of the structure of PSII reveals more than 50% of all hydrogen or proton positions within PSII. Moreover, they determine several previously undetected water binding sites.
Thus, the recent study illuminates the function of PSII in several key ways. Firstly, understanding the protonation states of subunits near the catalytic sites of PSII is essential for a comprehensive grasp of its catalytic function. Secondly, identifying the positions of water molecules within PSII clarifies the pathways that water takes through the system. Lastly, this study highlights the importance of employing various experimental techniques for structural analysis and emphasizes the power of cryo-electron microscopy.
This study has been published in Science: Hussein, A. Graça, J. Forsman, A. O. Aydin, M. Hall, J. Gaetcke, P. Chernev, P. Wendler, H. Dobbek, J. Messinger, A. Zouni, W. P. Schröder, Cryo–electron microscopy reveals hydrogen positions and water networks in photosystem II, Science, 2024, Vol. 384, Issue 6702, 1349-1355, https://doi.org/10.1126/science.adn6541