An important area of research at UniSysCat is the development of new catalysts for the production of hydrogen, the energy carrier of the future. With the help of electrocatalysis, hydrogen can be produced sustainably from renewable energy. This requires suitable electrodes, ideally without expensive and rare noble metals. Great hopes are pinned on heterostructured electrodes based on early transition metals such as iron and nickel - but how stable are they over time? A research team led by the UniSysCat group leaders Matthias Driess, Holger Dau and Ingo Zebger investigated different electrode materials and finds that these change in structure dramatically when operated for longer.
Using electrocatalysis, hydrogen is produced by splitting water. The efficiency of this process is mainly hampered by the unsatisfactory performance of the catalysts used for the oxygen evolution reaction (OER), one part of the water splitting process (2H2O -> 2H2 + O2) namely the generation of molecular oxygen (O2). Following the approach of avoiding noble metals, heterostructured oxyhydroxide electrodes have often achieved excellent performance for the OER. However, it has not been proven that the interfaces between the oxyhydroxide phases remain during the OER, especially when operated for prolonged times such as a day – which is a very interesting point for industrial applicability.
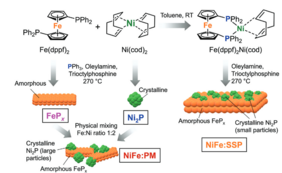
For this reason, the UniSysCat team led by Driess, Dau and Zebger has now systematically investigated how a series of heterostructures behave during prolonged operation: in total, eight electrodes made of four different nickel iron phosphide materials deposited on fluorine-doped tin oxide and nickel foam have been studied by in-situ Raman and X-ray absorption spectroscopy and high resolution elemental scanning electron microscopy. The eight electrodes were applied for the OER and their reconstruction was monitored after 24 h. The most active electrode was also applied under industrial OER conditions and for the value-added oxidation of alcohols to ketones.
The tests show a fairly stable performance of the electrodes over time, indicating that the active sites of the oxyhydroxides do not change significantly after their initial formation. However, neither the interfaces between the nickel and iron phases nor the morphologies of the precatalysts are preserved. Interestingly, the authors observe the mixing of the iron and nickel phases, resulting in iron-incorporated nickel oxyhydroxides.
This work suggests how dynamic materials behave under OER conditions and that heterostructures can be excellent OER electrodes even if their original interfaces do not persist and undergo restructuring.
The study has been published in Advanced Energy Materials: I. Mondal, J. N. Hausmann, S. Mebs, S. Kalra, G. Vijaykumar, K. Laun, I. Zebger, S. Selve, H. Dau, M. Driess, P. W. Menezes, The (In)Stability of Heterostructures During the Oxygen Evolution Reaction. Adv. Energy Mater. 2024, 2400809. https://doi.org/10.1002/aenm.202400809